Bacteria Antibody Candidate, TRL1068
Trellis Bioscience has used its CellSpot Technology to identify a high-affinity and broad-spectrum native human antibody, TRL1068, which targets and disintegrates bacterial biofilm. Biofilm is a physiological factor promoting
drug resistance in most clinically relevant multi-drug-resistant bacteria species, both gram positive and gram negative. Biofilm is also implicated as a factor promoting Alzheimer's pathogenesis.
TRL1068 has received more than $5M in grant funding from the NIH to facilitate preclinical development,
including multiple animal proof-of-concept experiments.
Bacteria
Broad-Spectrum Biofilm Disruptor Human mAb, TRL1068
Problem Scope
Antibiotic resistance is an increasing worldwide threat, with the rate of antibiotic resistance increasing across major bacterial species.
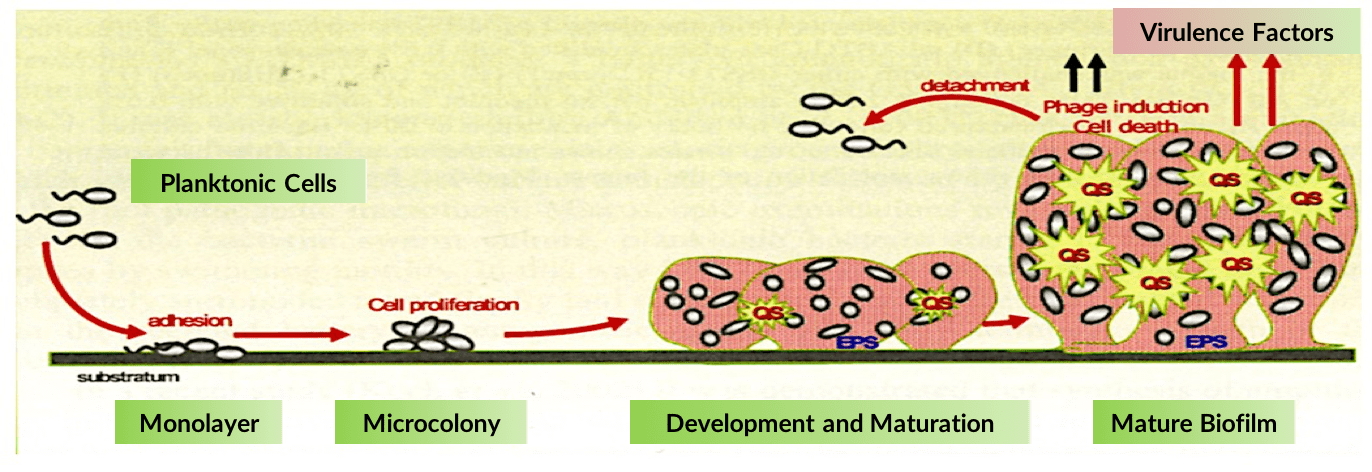
Bacterial biofilm, composed of proteins, polymers and embedded bacterial pathogens, is a leading cause of antibiotic resistance. Biofilm forms a protective matrix around multiple cooperative bacterial species thereby preventing the immune system from clearing an infection, while also substantially reducing the ability of antibiotics to kill the disease-causing bacteria.
The CDC estimates that ~75% of clinically significant infections are antibiotic refractory due to biofilm. ~60-80% of chronic infections involve biofilm, resulting in a $25B US annual cost.



Trellis Innovation:
High Binding Affinity and Broad-Spectrum
Trellis is pioneering a new approach by directly attacking the biofilm structure itself via a native human antibody, TRL1068, that extracts a key biofilm scaffolding protein. TRL1068 was discovered by screening ~5 million human B-lymphocytes using the CellSpot.
The epitope on the target protein is highly conserved across many bacterial species making the antibody effective against biofilm produced by a broad spectrum of clinically relevant pathogens, including all ESKAPE pathogens targeted as high-priority by the CDC and 10 out of the 12 WHO priority pathogens, impacting both gram-positive and gram-negative species.
Indications include: Infective endocarditis, osteomyelitis, diabetic foot infections, Lyme disease, cystic fibrosis, and many others. Similar to other Trellis antibodies, TRL1068 has subnanomolar binding affinity.
In several recent in vitro experiments, TRL1068 has demonstrated further ability to disrupt Borrelia burgdorferi spirochete biofilm,
implicated in Alzheimer's pathology. By BLAST analysis, TRL1068 also targets Treponema denticola, another spirochete species.


TRL1068 is a high affinity mAb that targets the DNABII protein family; BLAST analysis has found hundreds of DNABII homologs across many bacterial species. TRL1068 is broad-spectrum, disrupting biofilm across multiple gram-positive and gram-negative bacteria species. Upon biofilm disruption, bacteria regain antibiotic susceptibility.
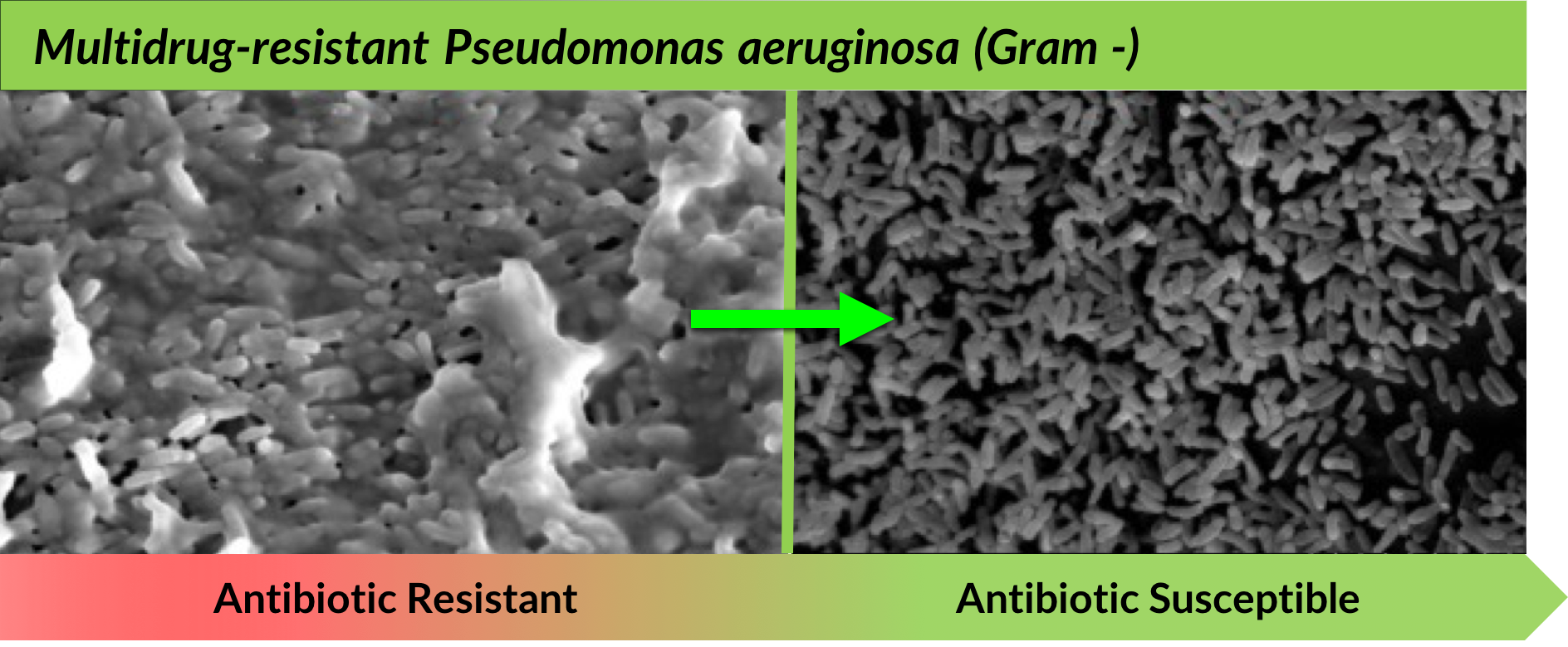
TRL1068 disruption of the biofilm matrix, in the absence of antibiotics, is captured below in scanning electron microscope (SEM) visuals:

In vitro experiments with scanning electron microscope (SEM) visuals.
Left: 4000X magnification; Treatment: 1.4ug/mL; TRL1068 for 12 hours.
Right: 2500X magnification, Treatment: 1.2ug/mL TRL1068 for 12 hours after 24 hours biofilm formation.

Animal Models:
Trellis has performed three animal models demonstrating efficacy of TRL1068 in potentiating antibiotic elimination of multiple species of multi-drug resistant bacteria.

#1: Murine Infectious Implant Model
In this experiment, Trellis demonstrated TRL1068 potentiation of daptomycin against methicillin-resistant Staphylococcus aureus (MRSA).
In a five day treatment study, a perforated, sterile teflon cage was implanted into mice and given 2 weeks to heal.
700 colony-forming unit (CFUs) of MRSA strain ATCC 43300 were injected into the lumen on Day 0 with biofilm forming in 24 hours.
Results: TRL1068 in combination with daptomycin (DAP) significantly reduced both planktonic and residual adherent MRSA bacteria.
Planktonic bacteria exposed to DAP + TRL1068 (15 mg/kg) became antibiotic sensitive (P=0.015) relative to DAP alone.
Cage-adherent bacteria exposed to DAP + TRL1068 were eradicated (P=0.04) relative to DAP alone.

Publication: Antimicrobial Agents Chemother. 60(4):2292-301 (2016) - Estellés, E., et al. Direct Link

TRL1068 increases efficacy of daptomycin (DAP), decreasing the number of planktonic (non-biofilm) bacteria by 100-fold.
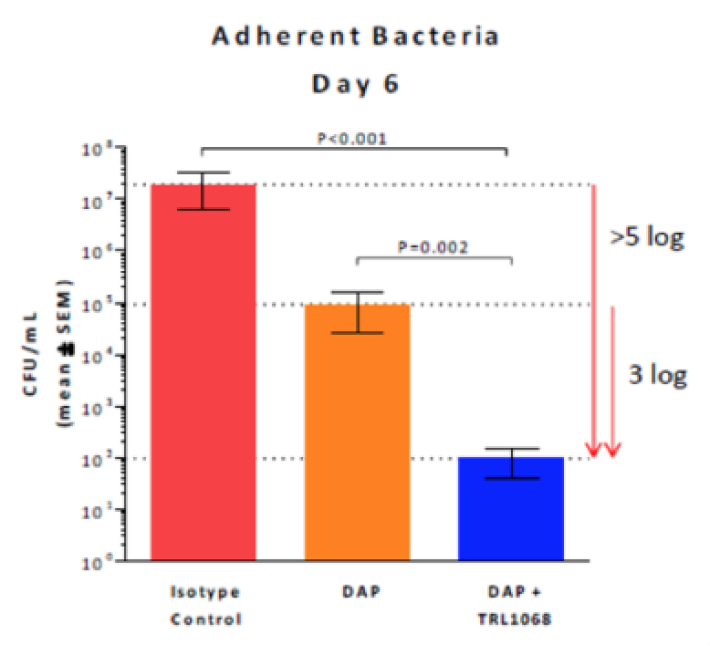
For biofilm-adherent bacteria, TRL1068 when combined with daptomycin (DAP) results in 1000-fold reduction of bacteria compared to DAP alone

#2: Infective Endocarditis Rat Model
In this experiment, Trellis demonstrated TRL1068 potentiation of vancomycin against methicillin-resistant Staphylococcus aureus (MRSA).
In this model, an indwelling catheter was used to scratch the heart valve surface. MRSA strain 300-169 (associated with clinical endocarditis and obtained from the blood of a patient with a persistant infection) was systemically introduced, which colonized the injured site and formed biofilm.
The mice were treated via intravenously for 5 days with vancomycin (VAN) + TRL1068, VAN alone, or isotype control.
Results: TRL1068 in combination with vancomycin (VAN) significantly reduced MRSA densities in biofilm vegetation relative to controls, as well as reducing propensity for septic metastases in kidney, spleen, and liver.
The combination also reduced mortality.
Planktonic bacteria exposed to DAP + TRL1068 became antibiotic sensitive (P=0.015) relative to DAP alone. Cage-adherent bacteria exposed to DAP + TRL1068 were eradicated (P=0.04) relative to DAP alone.



Publication: Antimicrob Agents Chemother 57(3):1447-1454 (2013) – Yan Q. Xiong, MD, PhD; Arnold S. Bayer, MD (UCLA). Direct Link

#3: Murine Soft Tissue Infection Model
In this experiment, Trellis demonstrated TRL1068 potentiation of imipenem against gram-negative Acinetobacter baumannii. Infection was induced by implanting a pre-colonized Teflon catheter segment (1 cm) inoculated with 106 CFU/catheter of A. baumannii strain HUMC-12. The mice were treated with imipenem alone or in combination with TRL1068. Imipenem alone and imipenem plus isotype control mAb were minimally effective in reducing A. baumannii bioburdens as compared to the untreated group.
Results: the increased exposure to imipenem in combination with TRL1068 showed a significant improvement in efficacy as compared to untreated and imipenem plus isotype control mAb (-1.8 and -1.6 log10 CFU/catheter, respectively; p<0.001).


Publication: Antimicrob. Agents Chemother. doi:10.1128/AAC.00904-17 (2017) – Yan Q. Xiong, MD, PhD. Direct Link
